eCRF
for Clinical Trials
We provide a flexible eCRF system for non-commercial clinical trials, configurable to any clinical trial study protocol.

Above all, the system was to be easy to use, user-friendly and extremely flexible.
The prepared solution meets all the required quality standards, and thanks to full control over the code, we can further develop and adapt it to the changing requirements of regulators and the clinical trials market.
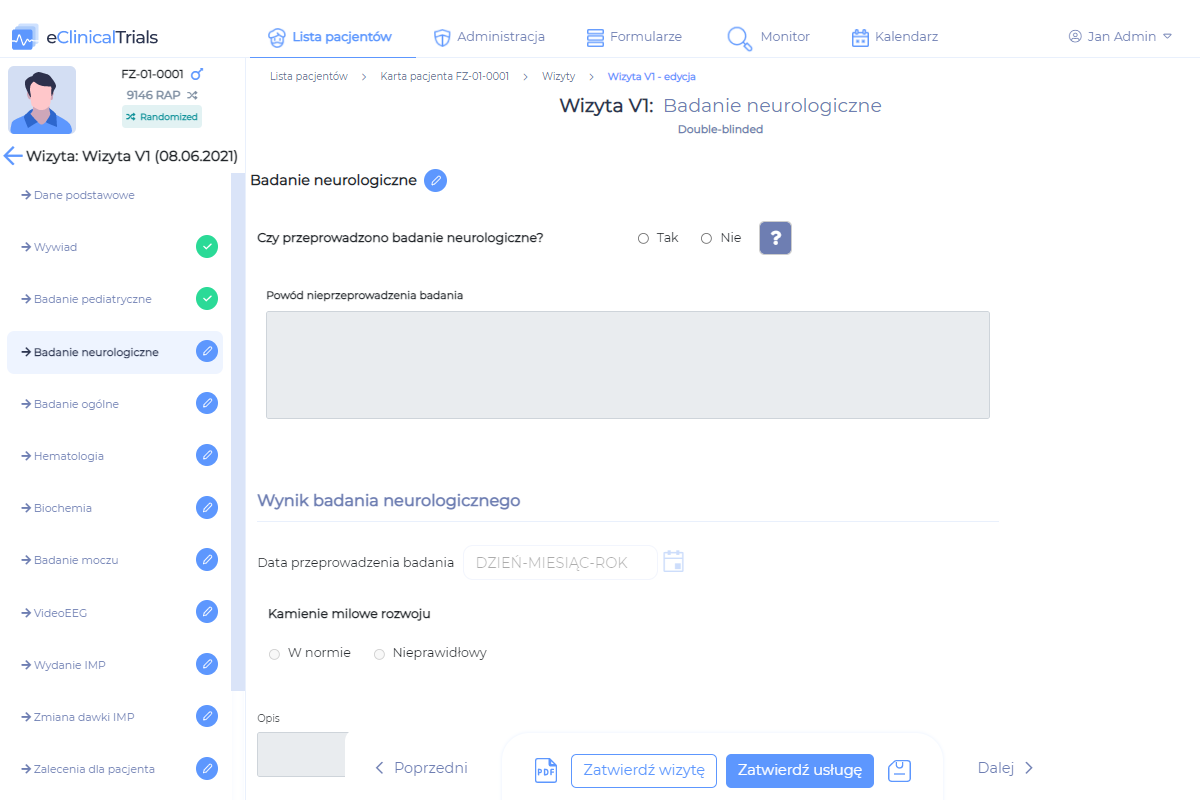
This module enables the integration of eCRF with HIS class hospital systems and automates the process of filling out examination documentation in terms of information previously entered as part of everyday activities at the patient.
eCRF facilitates the daily work in a clinical trial by taking care of all key processes.
eCRF provides:
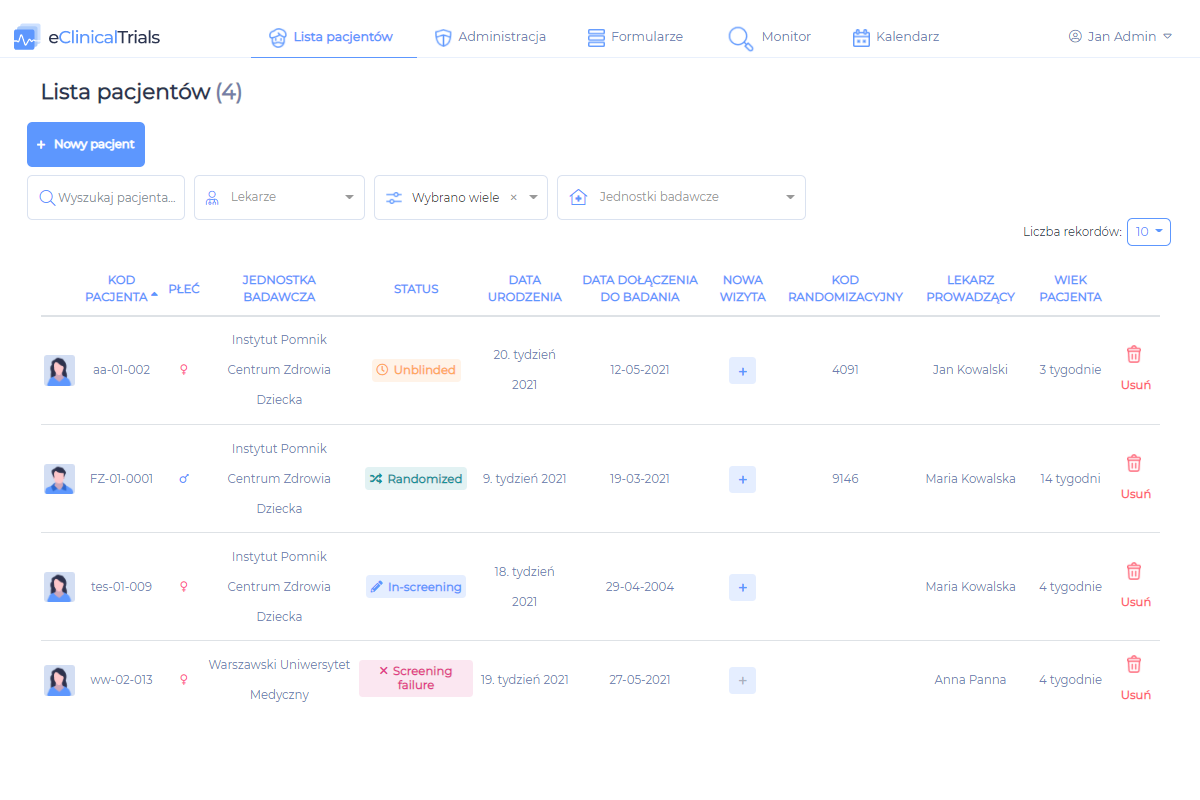
- data collection in full accordance with the study protocol
- facilitates data processing and supports the process of preparing analyzes and analytical models tailored to the needs of researchers
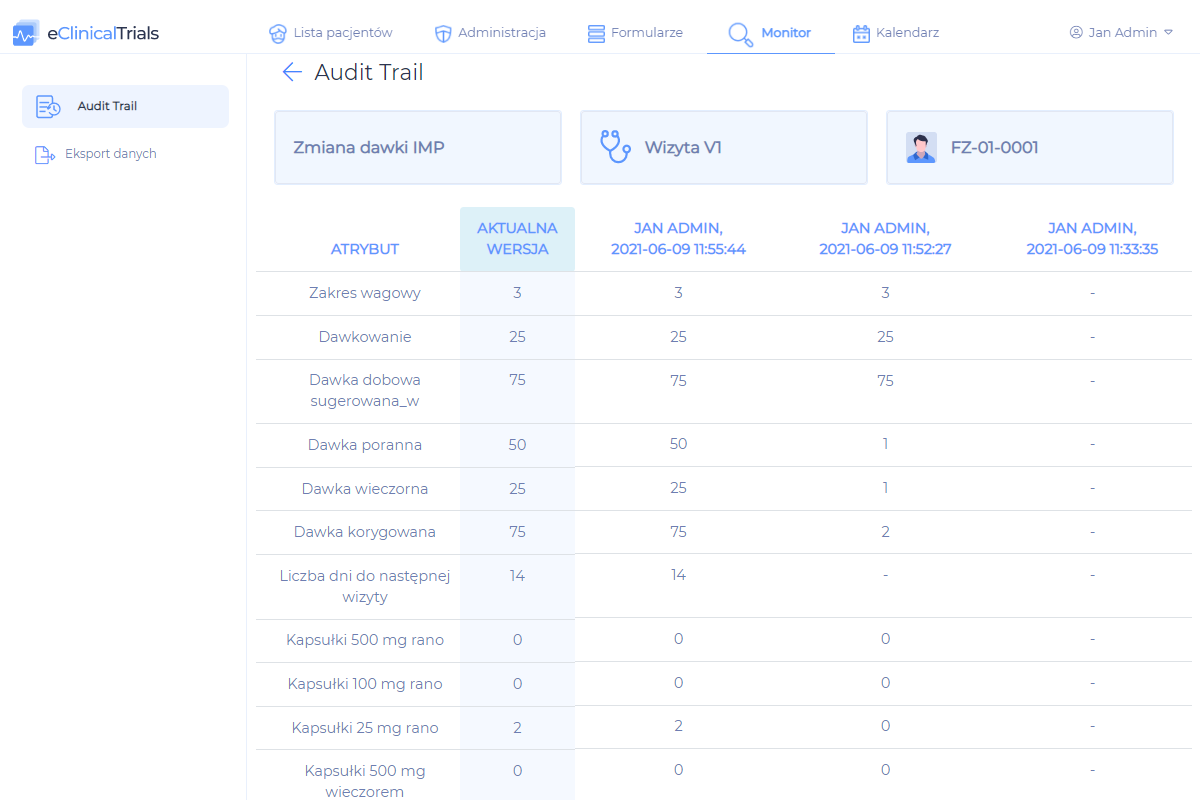
- ensures the security of data processing (Audit Trail)
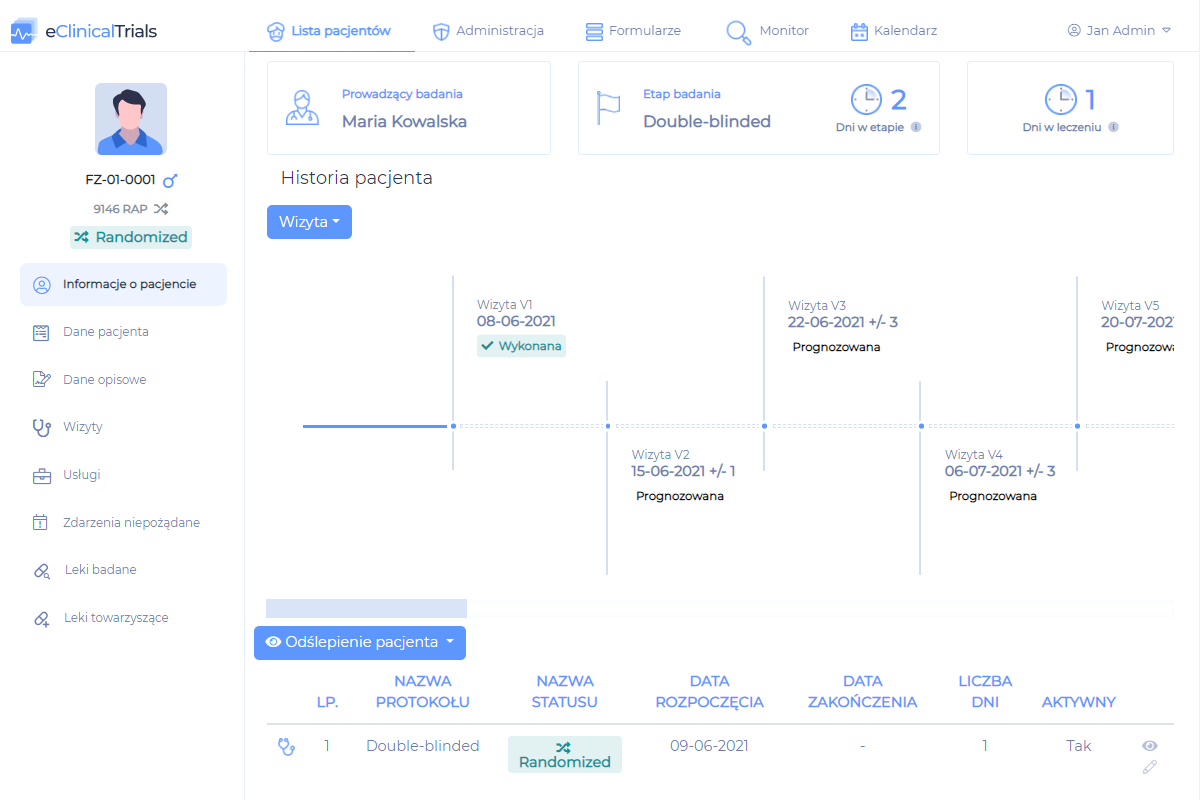
- efficiently facilitates the monitoring of the progress of the study
- ensures compliance with regulatory requirements for the collection of medical data
Our eCRF is part of our own IT platform, which focuses on combining a number of tools from the world of medical informatics into one complete system for handling clinical trials. Thanks to this, it is possible to quickly and conveniently connect data from the eCRF with the statistical, bioinformatic, genetic and other analysis module available on our platform.
More about eCRF solution at www.ecrf.com
Above all, the system was to be easy to use, user-friendly and extremely flexible.
The prepared solution meets all the required quality standards, and thanks to full control over the code, we can further develop and adapt it to the changing requirements of regulators and the clinical trials market.
This module enables the integration of eCRF with HIS class hospital systems and automates the process of filling out examination documentation in terms of information previously entered as part of everyday activities at the patient.
eCRF facilitates the daily work in a clinical trial by taking care of all key processes.
eCRF provides:
- data collection in full accordance with the study protocol
- facilitates data processing and supports the process of preparing analyzes and analytical models tailored to the needs of researchers
- ensures the security of data processing (Audit Trail)
- efficiently facilitates the monitoring of the progress of the study
- ensures compliance with regulatory requirements for the collection of medical data
- provides integration with the platform for managing a clinical trial center, also created by us.
Our eCRF is part of our own IT platform, which focuses on combining a number of tools from the world of medical informatics into one complete system for handling clinical trials. Thanks to this, it is possible to quickly and conveniently connect data from the eCRF with the statistical, bioinformatic, genetic and other analysis module available on our platform.
More about out eCRF solution at www.ecrf.com
Please contact us

www.ttsi.com.pl
office@ttsi.com.pl
tel.: +48 22 331 80 20
NIP: 527-294-32-09 | REGON: 387666213 | KRS: 0000872108
Copyright 2023

 MedStream Designer – Data analysis
MedStream Designer – Data analysis OncoReports – Medical registries
OncoReports – Medical registries Symptomo – Patient monitoring
Symptomo – Patient monitoring OncoDash – Data visualization
OncoDash – Data visualization eCRF – Clinical trials
eCRF – Clinical trials System integration
System integration Data Science
Data Science Support for clinical trials
Support for clinical trials
